How do snowflakes form? Are they really unique?
The genesis of snow at the micro level is a fascinating story that has important implications for society.

One of my favorite things about skiing is inspecting individual snowflakes as they accumulate on my jacket and pants while I’m riding a chairlift during a storm.
These moments are precious because they’re so rare. When it’s snowing, I’m usually sleeping, working, traveling, playing, or doing something else, and I can’t be bothered to examine the precipitation. On a ski lift, I’m a captive audience.
No, I don’t carry a magnifying glass on storm days so that I can study the snow crystals on my clothing. But even with the naked eye, you can sometimes get lucky and see the intricate patterns and symmetrical shapes that have somehow survived a long fall from the heavens to create one of nature’s most beautiful displays.
“How full of the creative genius is the air in which these are generated! I should hardly admire more if real stars fell and lodged on my coat.”
— Henry David Thoreau on snowflakes
In a previous post, I wrote about snow at the macro level and why it’s so important for weighty issues, including the water supply, biodiversity, recreation, jobs, and more. In this post, I want to take the opposite approach and discuss snow at the micro level of the flake.
En masse, miniscule snowflakes can paralyze entire regions during a major winter storm, so it’s natural to focus on the big picture and snow’s impact on the landscape. But I also think there’s a fascinating and important story at the other end of the spectrum, where you need to squint or use a lens to see what’s happening.
I used to think that snowflakes were like frozen raindrops and that all snowflakes more or less looked like those paper cutouts I brought home from arts and crafts.
But the real story is not only more complicated but also more interesting because the diversity of snowflakes explains their origins and why snow can look, feel, and behave so differently from storm to storm and from place to place.
In fact, a citizen science project, Snowflake ID, seeks to improve weather forecasting and climate change projections by employing machine learning to classify snowflakes. As the project notes:
. . . the Intergovernmental Panel on Climate Change (IPCC) has stated that accurate representation of the size, shape, and density of snow and ice particles is one of the most important factors for accurately predicting the amount of global warming for a given amount of increased greenhouse gases. Likewise, the exact type of snowflakes falling during a winter storm is directly related to how quickly the snow piles up and affects civilian life.
Snowflake generation: condensed version
The genesis of snow is a fascinating process.
First, let me distinguish between snowflakes and snow crystals. Here’s an explanation from SnowCrystals.com, a wonderful site by Ken Libbrecht, a CalTech physics professor and one of the world’s leading experts on snowflakes.
When people say snowflake, they often mean snow crystal. The latter is a single crystal of ice, within which the water molecules are all lined up in a precise hexagonal array. Snow crystals display that characteristic six-fold symmetry we are all familiar with . . .
A snowflake, on the other hand, is a more general term. It can mean an individual snow crystal, but it can also mean just about anything that falls from the winter clouds. Often hundreds or even thousands of snow crystals collide and stick together in mid-air as they fall, forming flimsy puff-balls we call snowflakes. Calling a snow crystal a snowflake is fine, like calling a tulip a flower.
If you have any interest in snow, I also highly recommend Ken Libbrecht's Field Guide to Snowflakes, a book with gorgeous images that also shows how to observe and photograph snowflakes.

Whether you call it a snow crystal or snowflake, snow begins in the atmosphere when water vapor condenses to form a crystal. A freezing cloud laden with moisture is a prerequisite for snowflakes, but so are the tiny particles, known as nuclei, upon which the water will freeze.
“Pure water droplets can be supercooled to nearly -40 degrees before they freeze,” Libbrecht writes. “Dust provides a solid surface to jump-start the freezing process, so dust-laden droplets begin to freeze at around -6 degrees.”
Here’s how meteorologist Matthew Cappucci explains the role of the nucleus.
Usually, that will be something like a pollen grain, dust particle or some other airborne bit. It could be smoglike aerosols or the volatile organic compounds released by plants. Even tiny soot particles or microscopic metal bits spewed in a car’s exhaust could become the nuclei around which snowflakes crystalize.
Indeed, when the air is very clean, it can be very difficult for a cloud’s moisture to find a nucleus.
The gist of “cloud seeding,” a technology meant to boost snowfall, involves creating more nuclei in clouds by shooting chemicals, such as silver iodide and dry ice, into the atmosphere.
Once a snow crystal is born, it grows as more water vapor condenses through two main processes: faceting and branching. NOAA’s SciJinks explains the difference:
A facet is essentially a flat face on a 3D shape, like a prism. They form naturally when a crystal grows. In ice crystals the shape they take mirrors the shape of the molecules forming the crystal. The crystal structure of frozen ice is a six-sided shape. Therefore an icy facet is six-sided as well. That is where the symmetry in a snowflake comes from.
The second way to grow a snowflake is to form branches. Not surprisingly, this is what creates those beautiful tree-like structures. Branches form because water vapor will condense on the first thing it touches. If there is a small bump on a flake’s surface, the vapor will condense there instead of traveling any further. Now the bump is bigger and even more likely to ‘catch’ water vapor at that point. The process repeats itself and a branch is formed!
Snow crystals can evolve into an impressive array of forms while passing through changing atmospheric conditions on their way to the ground. That journey can take anywhere from 10 minutes to more than an hour, according to Cappucci. While snow falls at between 1 and 4 miles per hour, air currents can carry the flakes back up, making for a circuitous journey.

Classifying snowflakes
Not every storm will produce a menagerie of iconic crystals on your ski pants with those dendritic, radiating formations worthy of a holiday card. In fact, scientists like Libbrecht have come up with dozens of categories to describe the diversity of shapes. The graphic below, from Libbrecht’s SnowCrystals.com, shows his classification scheme.
Beauty is in the eye of the beholder, but I’d venture to say that snow wouldn’t be so magical for so many people if it were solely made up of “solid columns” or “simple needles.” By far, “irregulars” are the most common type of snowflakes.
The different shapes are due to varying conditions in the atmosphere when the flake forms and grows, which is summarized in Libbrecht’s diagram below.

The horizontal axis at the bottom shows the temperature, while the vertical axis on the left charts a measure of humidity known as supersaturation. These two factors—temperature and humidity—are different in every weather system and can change dramatically on a snowflake’s tumble through the sky.
The science behind the graphic above, known as a snow crystal morphology diagram, was “discovered in the 1930s by Japanese physicist Ukichiro Nakaya and his collaborators,” according to Libbrecht. The diagram below is a classification by Nakaya, who is credited with creating the first artificial snowflake.
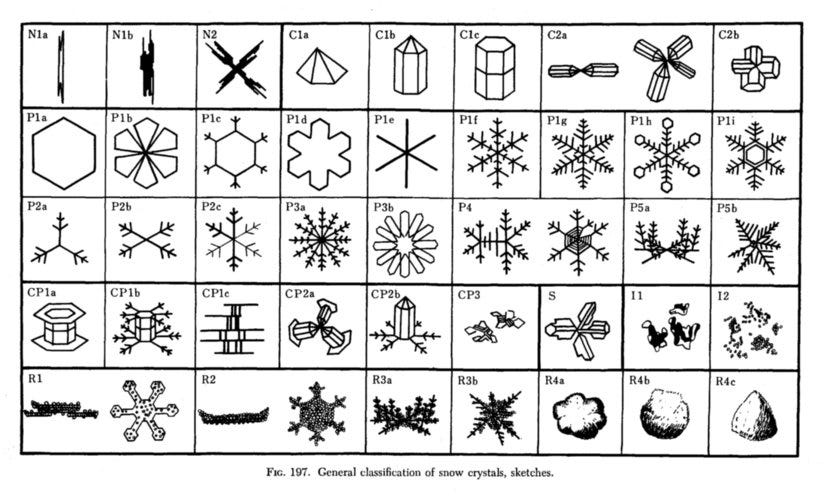
“Nakaya used to say that snowflakes are like hieroglyphs from the clouds,” Libbrecht writes, “because you can infer the conditions in the clouds by examining the shapes of the falling snow crystals.”
Are no two snowflakes the same?
Sadly, even the humble snowflake has become politicized in our partisan world. The word “snowflake” is now hurled as an epithet, as Wikipedia explains:
“Snowflake” is a derogatory slang term for a person, implying that they have an inflated sense of uniqueness, an unwarranted sense of entitlement, or are overly emotional, easily offended, and unable to deal with opposing opinions.
Collins English Dictionary described “snowflake generation” as one of its 2016 words of the year, defining the term as “the generation of people who became adults in or after the 2010s, viewed as being less resilient and more prone to taking offence than previous generations.”
Fragility and uniqueness lie at the heart of the “snowflake” insult. But while snowflakes can certainly be frail and fleeting, could you survive a 5,000-foot fall, not only intact but also while morphing into a symmetrical pattern?
And what about that age-old question: are snowflakes really unique?
“It's a funny question, almost like a Zen koan,” Libbrecht writes on a page dedicated to the subject. “If two identical snowflakes fell, my inquisitive friend, who would know? And can you ever be sure that no two are alike, since you cannot check them all to find out?”
What seems like a simple question has a convoluted answer. A “nano-snowflake” might only have a handful of molecules of H2O. “So there's a reasonable probability that two 10-molecule snow crystals would be exactly alike,” according to Libbrecht.
But that’s not what people have in mind when they think of snowflakes, which have a lot more molecules.
“A typical snowflake may contain 1,000,000,000,000,000,000, or one quintillion water molecules,” writes Cappucci. “Those building blocks can configure themselves in a virtually infinite array of patterns. So it stands to reason that no two snowflakes that you encounter will ever be exactly the same.”
Libbrecht agrees, writing, “it’s unlikely that any two complex snow crystals, out of all those made over the entire history of the planet, have ever looked completely alike.”
That’s the technical answer, but if we loosen our criteria a bit, snowflakes don’t appear as unique. If, for example, you compare very basic snowflakes by using a microscope, rather than examining the arrangement of their molecules at the atomic level, some of the shapes will look the same.
“Crystals with simple shapes often look similar to one another, and it’s not hard to imagine that if you sifted through a reasonable number of Antarctic snow crystals you would find two that were essentially indistinguishable in a microscope,” Libbrecht writes.
Moreover, Libbrecht has grown snow crystals in the laboratory that he says are akin to “identical twins.” If you view the video below from a distance, the two “designer snowflakes” look the same, but if you get close and study the fine details, you’ll see they’re not exactly alike.
When snowflakes turn into something else
Unfortunately, a precious, unique snow crystal high in the atmosphere may not survive in that state on its way to terra firma. As the flake falls, it may encounter warmer conditions that cause it to melt and turn into rain. Or the precipitation may be classified as the dreaded “freezing rain” if the drops turn to ice on cold surfaces at ground level. Another possibility is for the snow to melt on its way down, but then freeze again before reaching the ground, which is sleet. The graphic below from the National Weather Service summarizes how these types of precipitation differ.

While snow makes me and millions of other people happy, I don’t sense there’s a strong constituency out there for sleet and freezing rain, even though they do start as snow.
There’s one more type of precipitation connected to snow worth mentioning. Graupel, which is also known as “soft hail” and “snow pellets,” begins as snow, but the flakes pick up an extra layer of moisture known as rime on their way to the ground as supercooled droplets adhere to the crystals. (I have a longer discussion of graupel on SnowSlang.com, my skiing/snowboarding glossary and blog.)
To me, graupel looks like those tiny balls of Styrofoam that our dog recently spilled onto the floor by ripping open a bean bag chair. The microscopic image below shows a layer of rime growing on both ends of a columnar snow crystal.

Snowflakes, shoveling, and skiing
Anyone who shovels snow knows that each storm is different. Sometimes the snow is airy and easy to push around. Sometimes it’s dense and a pain to remove (but great for making snowballs).
The types of flakes that fall, the moisture content of snow, and other aspects of the weather can also make for diverse conditions on the slopes.
“Large dendrite snowflakes create the lightest, fluffiest powder for skiers and riders. Colder temperatures and a higher amount of moisture in the air are the conditions necessary for fluffy powder,” writes Bryan Allegretto, a forecaster at opensnow.com. “Because these snowflakes are ‘perfectly’ shaped with six points, they tend to stack on top of each other instead of packing tightly together. Stacked snowflakes ensure that the new snow is mostly made up of air pockets, and this is what creates the perfect snow conditions with light, ‘blower’ powder.”
“Blower” is skiing/snowboarding slang for snow that is so light and fluffy that you can scoop some in your glove and blow it out of your hand. Riding on that kind of snow feels like floating on air and is one of my life’s joys. Then again, surfing on creamy, denser snow is also fun!
Once snow is on the ground, a whole new story begins as the flakes morph, melt, evaporate, and sublimate (turning directly into water vapor without becoming a liquid.)
Those changes have big implications for avalanche danger, the water supply, local ecosystems, and much more, but I’ll have to wait until another day to delve into those issues.
In the meantime, I’m patiently waiting for the first storm of the season to deliver snowflakes that I can admire on my sleeve or deck. After writing this post, I’ve decided to invest $8.49 in a jewelry loupe so I can see the crystals more clearly and savor the experience.
Learn more
Below are some links to resources that I found helpful in learning about snowflakes.
SnowCrystals.com. Website by CalTech physics professor Ken Libbrecht.
Ken Libbrecht's Field Guide to Snowflakes. Voyageur Press, 2016.
“The Formation of Snow Crystals.” Ken Libbrecht, American Scientist, January-February 2007, Volume 95, Number 1.
“Who Ever Said No Two Snowflakes Were Alike?” Nicholas St. Fleur, The New York Times, January 22, 2016.
“Explainer: The making of a snowflake.” Matthew Cappucci, ScienceNews Explores, February 14, 2019.
“How classifying snowflakes could help forecast weather.” Kate Ravilious, The Guardian, January 20, 2022.
“How do snowflakes form? Get the science behind snow.” National Oceanic and Atmospheric Administration, December 21, 2022
“How Do Snowflakes Form?” NOAA SciJinks, undated.
“Snowflakes - Explained.” Bryan Allegretto, opensnow.com, April 5, 2020.
“Snowflakes as big as frisbees?” William J. Broad, The New York Times, March 20, 2007.
“Wintry precipitation types can be confusing. Here’s what they are and how they form.” Matthew Cappucci and Ian Livingston, The Washington Post, November 14, 2018.





Loved this, Mitch! And I'm so glad you dug into what a "graupel" is, since that one leaped out at me as I scrolled down, and it seemed worth going down as a whole separate rabbit hole, with a fascinating word derivation, etc. It appears that maybe the Germans are the ones with dozens of different words for snow (although I imagine the Inuits have a bunch, too, as the old saying goes).
As a Maine native, I thought I knew all about snow. Thanks, Mitch, for the page turner (page scroller?) that explains a big part of my childhood experience. I am now going to wow my friends with my new knowledge on snow crystals, and I finally know when to volunteer to shovel (I’m signing up for the “large dendrite” shift.) Great writing; I’m looking forward to more Snow News!